- Board of Officers +
- Committees +
-
Calendar
-
News
-
Agenda Requests
- Resources +
-
Gallery
-
Contact
- Board of Officers
- Committees
-
Calendar
-
News
-
Agenda Requests
- Resources
-
Gallery
-
Contact
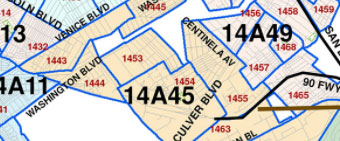
Monday: Sustainaability and Beautification Committee Meeting
Posted on 03/05/2026

Sustainaability and Beautification Committee Meeting
Virtual Only
Files
It's Your Venice, Get Involved!
The Venice Neighborhood Council is made up of individuals from our community who
are interested in improving and maintaining the quality of life of the stakeholders of Venice.